Solvent Effects in the
Reaction of 5'-O-Benzoyl-2,3'-AnhydroThymidine with Dimethylammonium azide:
Kinetics Analysis and Ab Initio
Calculations
Elena
V. Korchevskaya1,
Alexander Yu. Steblyanko2,
Alexander A. Malin1,
Mikhail B. Shcherbinin, Vladimir
A. Ostrovskii1
1Saint-Petersburg State
Institute of Technology, 198013, St.-Petersburg, Moskovskii pr., 26, Russia
2Department of Polymer
Science & Engineering, Faculty of Engineering, Yamagata University, 4-3-16
Jonan, Yonezawa, Yamagata 992-8510, Japan
The key step of the process for preparation of the important anti-AIDS
drug, 3'-azido-2',3'-dezoxythymidine (AZT), is the reaction of a semi-product
5'-O-benzoyl-2,3'-anhydrothymidine (I)
with azide agents of various nature.
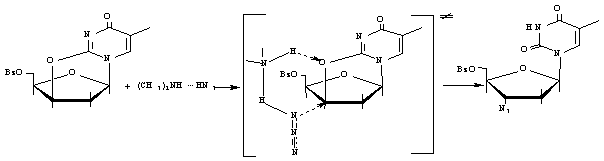
In this work a plausible mechanism for the reaction of
5'-O-benzoyl-2,3'-anhydro thymidine (I)
with dimethylammonium azide (II) in
a binary, DMF-1,4-dioxane, solvent system (Scheme) was investigated by means of
kinetics studies and ab initio MO
computations (GAMESS, RHF/STO3-21G basic set). The suggested mechanism involved
the cyclic activated complex (III)
formation on the key step of the process. Due to this, the simultaneous
protonation of the oxygen atom O2 and nucleophylic addition of azide
group with consequential C3'-O2 bond cleavage became
possible.
II III I

![]()
It was found in our kinetics experiments that the addition of low polar
dioxane increased the rate of the reaction of I with II. However, the
dependence of this rate from the Kirkwood’s function was shown to be nonlinear.
When a significant amount of dioxane was added it was observed that the
experimental plot deviated to the values lower than was expected. This was
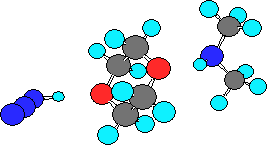
explained by the existence of the effect of specific solvatation. In fact, our
model calculations showed that the addition of dioxane to the reaction mixture
led to the formation of the dixane complex with a nucleophylic agent II with spatially separated amine and
azide parts (Figure) and interrupted the formation of III.
This work was by the RFBR (grant no 01-03-32531)