Abstract: MATH/CHEM/COMP 2002, Dubrovnik,
June 24-29, 2002
|
|
Modelling
of catalytic reactions in {CO2+H2+NH3} system Sławomir Ostrowski, Michal H. Jamroz, and Marek A. Borowiak Industrial Chemistry Research Institute, Warsaw, Poland In
the field of modern industrial catalysis one of a problem is to search for
high selective catalysts for the reactions to mono-alkylated amines. The goal
of this work is to show application of our new tool for design of catalysts -
an Impulse Oscillation Model (IOM)1 - for the catalytic reactions
in {CO2+H2+NH3} system. The system was experimentally
studied by Baiker et al.2,
where was shown among Cu, Ag, Ni, Pt, Co, Fe metallic catalysts supported on Al2O3 the Cu-Al2O3
is the most selective one to mono-alkylated amines. In
the IOM model basically IR vibrational modes
of the reactants and catalytic center are regarded as parameters, which are
assumed to be a representation of the electron density distribution in the
system. For assumed type of catalyst (e.g., metal oxides or metals supported
on oxide etc.) and reaction mechanism, we search for the frequencies of the
reactants and catalytic center synchronization, by a computer program, to
obtain optimal conditions for the reaction to occur. As results of
computation we obtain a set of the ranges of the wavenumbers where the vibrational modes of the catalytic center should appear. It
makes possible to assign the vibrators to a particular catalytic system or at
least to reduce the number of possible catalysts. We
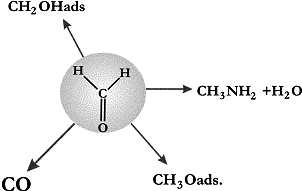
present result of the IOM modelling for the following subsystem
Our
computation selects the copper catalysts as the best for the reactions to
mono-alkylated amines in the system. It fully agrees with the experimental
results2. This work is part of the COST D9/0012/98
supported in Poland with KBN grant No 3T09B 05017. 1 M. A. Borowiak, J.
Mol. Cat. A: Chemical 156
(2000) 21-57. 2 S. V. Gredig, R. A. Koppel, A. Baiker, App. Catal. A: General 162 (1997) 249-260. |