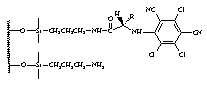Abstract: MATH/CHEM/COMP 2002, Dubrovnik,
June 24-29, 2002
|
||||||||||||||||||||||||
|
EXPERIMENTS AND MODELS IN ENANTIORECOGNITION BY CHIRAL STATIONARY PHASES
Darko KONTREC, Vladimir VINKOVIC, and Vitomir SUNJIC Rudjer
Boskovic Institute, P. O. Box 180, 10002 Zagreb, Croatia Aesthetically motivated supramolecular synthesis based on
symmetry, topology, and network properties represent a distinct aim for
subjects such as crystal engineering or chiral recognition. Enantiorecognition represents a bottom line
process which enables separation of enantiomers by chromatography on chiral
stationary phases (CSPs).
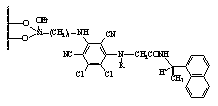
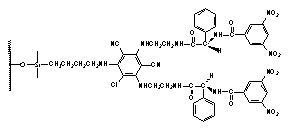
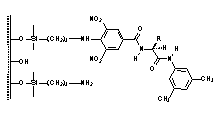
I II
III
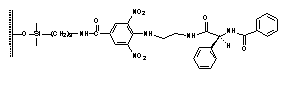
IV V CSPs based on assemblies of planar, homo- or
heteroaromatic branching units, usually with one or two chiral subunits and a
spacer that enables binding to silica gel, are known as Pirkle-type or
brush-type CSPs and are repeatedly reported.1-3We entered the field
of enantioseparation by developing two main groups of CSPs; the first group (I-III) is based on derivatives of
persubstituted, p-basic aromatic ring,4-6
the second CSPs (IV-V) are derived
from 4-substituted 3,5-dinitrobenzoic acid derivatives, which serve as the p-acidic branching unit.7 Rationale behind the synthesis of over 50
representatives of CSPs I-V, and
correlation of their enantioseparation ability to their conformational and
structural properties, in particular H-bonding ability and p-acid/base interactions, will be presented.
Separation of specific groups of racemic analytes, as e.g. a-aryloxypropionic acids, 4-substituted
1,4-dihydropyrimidines, and 1,4-benzodiazepines is achieved with specifically
designed CSPs; mechanisms of enantioseparation, and the observed
enantiomerization of configurationally unstable analytes will be discussed. 1 W. H.
Pirkle, J. Chromatogr. A 749 (1996) 19-24. 2 S. Allenmark, V. Schurig, J. Mater. Sci. 7 (1997) 1995-1963. 3 E. R. Francotte, Chimia 51 (1997) 717-725. 4 I. Novak, B. Kovac, D. Kontrec,
V. Sunjic, J. Electron Spectr. 199
(2000) 281-286. 5 D. Kontrec, V. Vinkovic, A. Lesac, V. Sunjic, A.
Aced, Enantiomer 5 (2000) 333-344. 6 a D. Kontrec, A. Abatangelo, V.
Vinkovic, V. Sunjic, Chirality 13
(2001) 294-301; b
V. Vinkovic, D. Kontec, V. Sunjic, L. Navarini, F. Zanetti, O. Azzolina, Chirality 13 (2001) 581-587; c A. Abatangelo, F. Zanetti, L. Navarini, D. Kontrec, V:
Vinkovic, V. Sunjic, Chirality 13
(2001) 984-992. 7 B. Zafirova, D. Kontrec, V. Vinkovic, V. Sunjic, Chirality (2002) submitted. |