Abstract: MATH/CHEM/COMP 2002, Dubrovnik,
June 24-29, 2002
|
||||
Correlation
of All pK Values
of Polycarboxylic Acids With Atomic Charges
Ivan JuraniC1, Zeljko Vitnik1, Branko DrakuliC2, Lidija Pfendt1,Gordana PopoviC3, and Branka DraZiC1 1Faculty of Chemistry, University of Belgrade, POB 158, 11001Belgrade, Yugoslavia 2Center for Chemistry IChTM, Njegoseva 12,
Belgrade, Yugoslavia 3Faculty of Pharmacy, University of Belgrade, Yugoslavia There are reports on the use of calculated electrostatic charges on
carboxylic hydrogen for the estimation of pKa values of carboxylic acids.
Various approaches could be found in the literature. Correlation has been
made with charges on carboxylic hydrogen as well as with the overall charge
on COOH group. Most of the mentioned reports comprise AM1 and PM3
semiempirical MO methods. The PM3 parameterization, with simulation of
dielectric solvent was used in our work as a reliable method for atomic
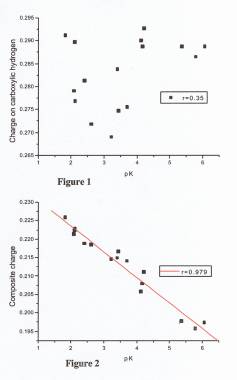
charges. The estimation of pK values of polycarboxylic acids by the correlation
with hydrogen charges was proven unsuccessful (Figure 1). Acids studied in
this work were: epoxy maleic acid, epoxy fumaric acid, epoxy citraconic
acid (2-methyl maleic acid), epoxy itaconic acid (2-methylene succinic acid),
epoxy mesaconic acid (2-methyl fumaric acid), epoxy-cis-aconitic acid
(2-carboxymethyl maleic acid) and epoxy-trans-aconitic acid
(2-carboxy-methyl fumaric acid). Correlation involves all pK values of epoxy di- and
tri-carboxylic acids, i.e., including first, second and (eventual)
third dissociation. As a result of the polylinear fit the composite
charge for carboxylic group is calculated as a weighted sum of atomic
charges, according to the formula: Q = qH + A*qO- + B*qC
+ C*qO= + D
|